
Infectious Bronchitis
Authors
Mark W. Jackwood, PhD
Dr. Mark Jackwood is Senior Technical Advisor for CEVA Animal Health in the United States. Previously he was the Head of the Department of Population Health in the College of Veterinary Medicine, at the Poultry Diagnostic and Research Center, University of Georgia, Athens GA (retired September 2022). Dr. Jackwood is a molecular virologist, and his primary areas of expertise are poultry respiratory viruses particularly avian coronavirus infectious bronchitis virus (IBV). Dr. Jackwood has published on the use of molecular techniques for the identification, characterization, and control of that virus. He also has studied genetic diversity, mutation rates, and evolutionary trends among coronaviruses to elucidate mechanisms that can lead to the emergence of new viruses capable of causing diseases in animals and humans.
Marco-Aurelio Lopes
Dr. Marco Aurelio Lopes is Poultry Corporate Director for the range IBD (Gumboro) and Infectious Bronchitis (IB) for CEVA Animal Health, based in France. He is Veterinary graduated in University of Sâo Paulo, Master degree in Avian Pathology. Dr. Marco has experience working in the Poultry industry production in broilers, breeders, GP, Hatchery and slaughterhouse.
Introduction and History
Avian infectious bronchitis (IB) is a highly contagious upper-respiratory disease of chickens. It was first described in 1931 in the USA and is now world-wide in distribution causing millions of dollars in losses to the poultry industry annually. The disease is caused by avian coronavirus infectious bronchitis virus (IBV) a gamma-coronavirus that can affect the respiratory, urinary, and reproductive systems of chickens, causing different disorders depending on the tissue tropism characteristics of the invading viral strain.
IBV Types
The spike glycoprotein located on the outside of the virus particle, is made up of S1 and S2 subunits The S1 subunit makes up the distal portion of spike and is unique for each different IBV type. Spike is involved in attachment and entry into the host cell, and the immune response to the infection is largely against spike. There are numerous different IBV types around the world and countless variant viruses stemming from mutations that can occur in the gene that codes for spike.
Clinical Signs of IB and Transmission
The incubation period of IBV is relatively short, 1-3 days. Chickens of all ages are susceptible to infection and morbidity rate typically reaches 100% for this highly infectious virus. Mortality is variable depending on the severity of secondary infections. The disease is most severe in hatchlings and chickens up to 3-4 weeks of age. Clinical signs are watery eyes, gasping, coughing, mucus in the nares and trachea and tracheal rales. When the kidneys are affected, flushing is often observed. In hens, a drop in egg production with misshaped eggs and wrinkled eggshells can be observed and infertility has been observed in breeding males. In addition, when female chicks are infected within the first 2 weeks of life, damage to the immature oviduct can occur resulting in false layer syndrome.
IBV is transmitted from infected to susceptible bird by aerosolization via the ocular/nasal route and to a lesser extent fecal/oral. The virus can spread very rapidly in a chicken house. One report found the reproductive number (R0) for IBV to be ~19 (de Wit et al. 27:464-471. 1998), meaning that 1 infected chicken can transmit the virus to 19 susceptible pen mates. The similarity of IB clinical signs to other poultry upper-respiratory tract diseases and rapid spread of the infection, make diagnosis of Infectious Bronchitis one of the important components of a control strategy for the disease.
Economic Impact of IBV
The losses from IBV infections are significant and can occur in the field and at processing. Losses in the field include uneven growth, poor feed conversion, extended time to reach market weight, mortality, related medication costs from secondary opportunistic bacterial infections, drops in egg production and egg quality, and reduced fertility in breeding males. Losses at processing include partial or total condemnations of the carcass, as well as the economic impact associated with a decrease in the speed of the production line.
Diagnosis
Serology, virus isolation, hemagglutination inhibition and virus neutralization have historically been used to identify and characterize IBV. In some cases those tests are still useful. However, highly sensitive and rapid molecular biology techniques, are now widely used to detect IBV directly from clinical samples. Type specific real time RT-qPCR (reverse transcription- quantitative polymerase chain reaction) tests have been developed for the most common IBV types circulating around the world, including vaccine types (Mo et al J. Virological Methods, 276, 2020, 113773)). However, IBV type specific real time RT-qPCR cannot distinguish between field and vaccines of the same type, nor can it identify IBV types outside the specific types it was designed to detect. Consequently, sequencing the S1 gene is used to obtain a more granular identification of IBV types. Sequence of the S1 gene can be used to group like virus types into clades as well as follow the natural course of viral evolution associated with IBV strains.
Evolution of IBV Strains Globally
IBV exists as multiple antigenic variants. These have been characterized as serotypes based on in vitro virus neutralization tests, as genotypes based on the S1 glycoprotein sequence and as protectotypes based on vaccine-challenge studies in chickens. In 2016, the virus was classified into 6 genotypes comprising 32 different lineages (Valastro et al. Infection, Genetics and Evolution 39:349-364, 2016) and since that publication, several additional genotypes/lineages have been reported.
Coronaviruses including IBV evolve by genetic drift, the accumulation of mutations in the viral genome over time. Much less frequently they evolve by genetic shift, which is recombination between two different IBV types (Jackwood et al. Infection Genetics and Evolution 12, 1305-1311, 2012). Viral antigenic variation and the potential emergence of new variant IBV types occurs when mutations become fixed over time in the spike glycoprotein gene. This can occur as the virus replicates in the host making it extremely important to control viral replication and transmission with vaccines whenever possible.
Vaccination and Vaccine Takes Against IBV
Vaccination is critical for the control of IBV, and both live and inactivated vaccines are used. Live vaccines are attenuated strains of the virus that are typically applied by spray or gel at 1-day of age and mostly stimulate local upper-respiratory tract immunity. Live vaccines are also sometimes given as a booster around 14 days of age in areas where IBV challenge is high or where multiple IBV types are circulating. Inactivated oil-adjuvant vaccines against IBV require individual application, usually by subcutaneous or intramuscular injection. These are used as booster vaccines to protect long lived birds (layers and breeders), as well as provide a source of maternal antibodies to breeder progeny. Killed vaccines are usually given multiple times throughout the pullet stage and are available as polyvalent vaccines that can also include NDV, IBDV and/or other poultry pathogens.
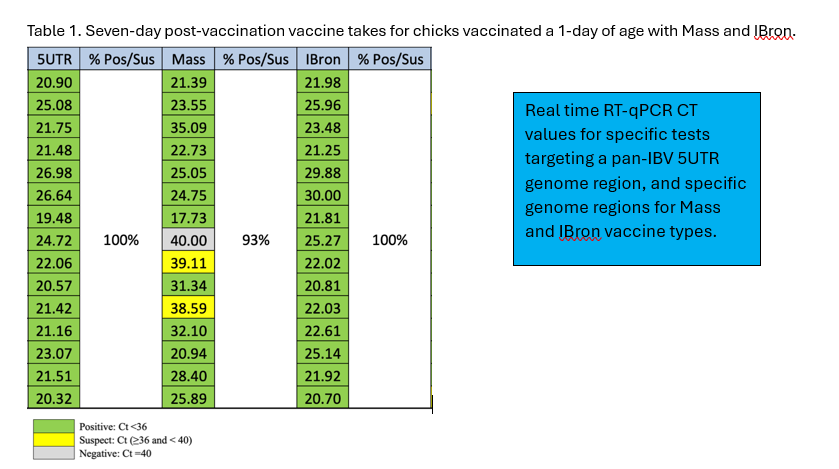
When using live attenuated vaccines, it is important to document that they are applied correctly. Proper storage and handling of the vaccines are critical since the enveloped virus is extremely sensitive to heat, disinfectants, contaminants in the water or gel, and mechanical damage from improperly working equipment. It is highly recommended that evaluating vaccine takes be part of the quality control vaccination program. For IBV vaccination, vaccine takes are monitored by collecting at least 15 choanal swabs at 5-7 days post-vaccination per house and testing them for the presence of the vaccine virus by specific RT-qPCR. Choanal swabs can be collected without harming the chicks and good vaccine takes should have 85%-100% of the samples positive for all IBV vaccine types applied (see Table 1).
Vaccination Against Multiple IBV types
Facing the constant changes in IBV strains, and the need to protect susceptible poultry populations, it is ideal to have vaccines that induce strong protection against the circulating virus. However, the enormous number of different IBV types, makes it a very challenging task – virtually impossible – to develop a specific IBV vaccine against each circulating type. In addition, developing and licensing a vaccine is a time-consuming process that can take from 3 to 5 years, at which time that virus type may no longer be present in the field.
Nonetheless, it is possible to combine IBV vaccines, with different antigenic characteristics, to obtain a synergistic effect and therefore broaden the spectrum of protection. In fact, it has already been demonstrated that combining antigenically different strains, such as those from the 793B group with a Mass type strain, acts synergistically to increase the protection spectrum of the two single vaccines if applied separately (Cook et al. Avian Pathology 28:477-485, 1999). And it has been shown that chickens can develop a protective immune response to as many as 4 different vaccine types when given simultaneously (Jackwood et al. Avian Pathology 49:335-341, 2020). Thus, a sound control strategy for IBV includes a combination of vaccines that will provide a broad spectrum of protection against the current circulating strains as well as new strains that may emerge.
Biosecurity and Management
Because IBV is highly infectious, vaccination in extremely important to control the disease. However, biosecurity and management procedures that comply with good practice standards accepted by the poultry industry does play a role in the overall control of IBV in chickens. Production systems that apply the “all-in, all-out” principle followed by sufficient down time between flocks, cleaning and disinfection of facilities, equipment, tools and transport vehicles can have a significant impact on control of the disease because it reduces the amount of the field virus(es) present. Multi-age poultry farms present a very difficult situation for control because older birds can be a source of the virus for younger birds. When possible, different age groups should be housed separately. Since IB is caused by a virus, there is no treatment for the disease. Antibiotics can be used to reduce the effects of secondary infections caused by bacteria. However, control through biosecurity and a sound vaccination program is the only successful strategy.
Summary and Final Thoughts
Avian coronavirus IBV is a highly contagious virus that causes severe economic losses to the poultry industry. The disease can affect the respiratory, urinary, and reproductive systems of chickens, causing different disorders depending on the tissue tropism. Like all coronaviruses, IBV replicates rapidly with a high mutation rate leading to multiple antigenic types and variants of the virus. Vaccination is critical for control, but individual vaccines for all the different IBV types are not practical. Two or more IBV vaccine types are typically given which acts synergistically to increase the protection spectrum. Knowing which vaccine or combination of vaccine types are effective against viruses circulating in the area is extremely important. Molecular diagnostic tests are available and essential to specifically sort out which field viruses are present.
Continuous efforts to characterize field isolates of IBV emerging from broiler, layer or breeder operations in different countries enable the drawing of epidemiological maps on the geographical distribution of the numerous IBV strains present around the world. Constant advances in these highly sensitive and specific methodologies enable poultry veterinarians to better understand the evolution of IBV in different parts of the world and identify the numerous IBV strains and variant viruses in the field. As new IBV strains are generated over time, some of them disappear in a short period of time and some of them prevail and spread to cause disease in other regions of the world. The mystery behind this behavior remains elusive making control extremely difficult. But, today, we have state of the art diagnostic tools and innovative molecular techniques to develop new vaccines for control of the virus.
 POULTRY
POULTRY